Study: Leg Pain From
A Herniated Disc
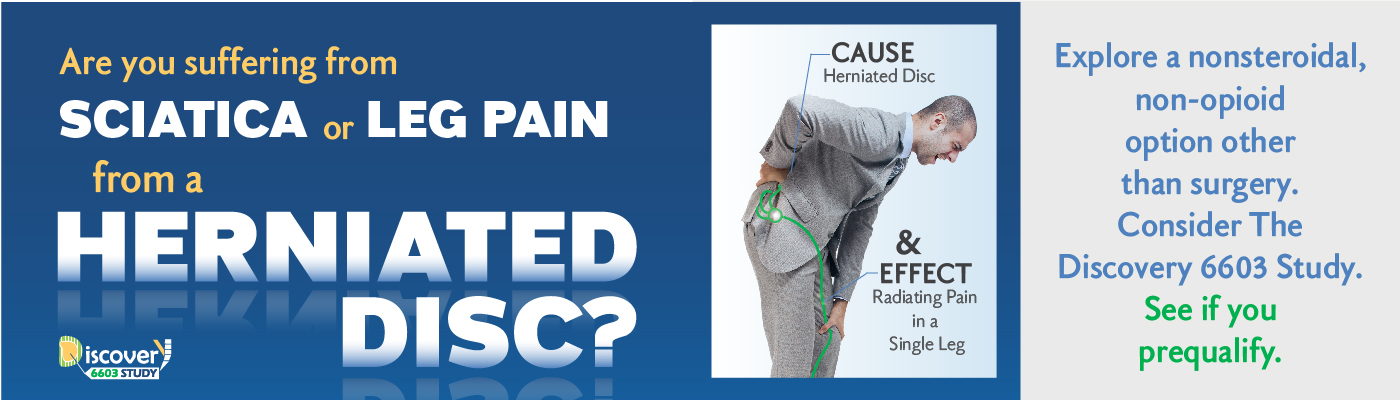
Are you experiencing sciatica, represented by leg pain, from a herniated disc?
Our clinic is conducting a clinical research study to examine the effectiveness of an investigational drug injection in reducing leg pain when compared with a control injection (an injection with no medication).
Call Us: 702-401-7318
or fill out the form to inquire about participating
Study: Leg Pain From A Herniated Disc
Are you experiencing sciatica, represented by leg pain, from a herniated disc?
Our clinic is conducting a clinical research study to examine the effectiveness of an investigational drug injection in reducing leg pain when compared with a control injection (an injection with no medication).
Call Us: (702) 401-7318
or fill out the form to inquire about participating
ABOUT HERNIATED DISCS AND LEG PAIN
Your lower back is made up of five lumbar vertebrae (bones) which surround and protect your spinal cord. Nerves travel along your spinal cord, sending information back and forth from your brain to other parts of your body. In between the vertebrae are flat, flexible discs that act as shock absorbers when you walk, run or jump. These discs have a flexible outer ring and a soft gel-like center.
When a disc’s gel-like center pushes through a weakened area of the disc it is called a herniated disc (or a slipped or ruptured disc). The gel may place pressure on the spinal cord or spinal nerves. This “pinches” the nerve and can lead to sciatica represented by the leg pain associated with herniated discs. A herniated disc happens most often in the lower back and is one of the most common causes of lower back and leg pain.
Current Treatments
Common treatments for herniated discs include:
- Medications such as:
– Pain relievers (acetaminophen, ibuprofen, naproxen)
– Nerve pain medications (Neurontin, Lyrica, Cymbalta)
– Muscle relaxants (Flexeril, Amrix, Lioresal) - Cortisone injections
- Physical therapy
- Surgery
STUDY LOCATION
Physicians Research Options, LLC
501 S. Rancho Dr., Suite D-26
Las Vegas NV 89106
Phone: 702-401-7318
Principal Investigator:
Daniel Burkhead, MD
Phone: 702-401-7318
About the Study:
About 320 people in the United States will take part in this clinical research study. The active study drug is administered as an injection and is being compared with a control injection (an injection that contains no medication). One out of two participants will receive the active study drug.
Study Criteria:
- 30 to 70 years of age.
- are experiencing leg pain associated with lumbar disc herniation, which emerged within the past 1 year
- are NOT receiving worker’s compensation
- are NOT in routine use of opioids/cannabis
are less than 35 of BMI
What Will Study Participants be Asked to Do?
The first step will be to meet with a member of the study team at your local research center to review an Informed Consent Form. This form fully explains the purpose of the study, possible risks and benefits and what will be expected of you as a participant. No procedures will begin until you have had all your questions answered and have signed this form signifying your understanding of its contents and your agreement to take part.
Participants in this clinical research study will receive one injection of the study drug (or a control injection). Involvement in the study will be about 12 months, during which participants will be asked to attend up to 11 study visits. You may be compensated for your time and travel.
Throughout the study, staff will monitor your health. They will collect blood and urine samples for laboratory testing. Participants will also receive an MRI and X-Ray. You will also be asked questions about leg-pain, medications being taken, and any side effects that you might have experienced. You will be provided with an electronic device on which you can record your pain condition and medication use on a daily basis.
Clinical research studies are designed to answer specific questions about study drugs and are needed to help find new and possibly better future treatments for diseases.
A study drug goes through several phases of clinical research to evaluate its safety and effectiveness. Depending on the results of the research, the study drug could later be available to the public.
Clinical research studies are performed in accordance with strict governmental guidelines. These guidelines help ensure that the study participant’s safety and rights are protected during the clinical research study, while allowing valuable information about the study drug to be collected.
Volunteering to participate in a clinical research study is an important decision that should be thought through carefully. Participation is entirely voluntary and an individual has the right to decline participation or to discontinue participation at any time, for any reason, without penalty or loss of any of the benefits to which they would otherwise have been entitled.
About PRO
Physicians’ Research Options (PRO) is an experienced Clinical Research Company dedicated to the performance of Phase II, III and IV Pharmaceutical and Pilot/Pivotal Medical Device clinical trials. Our staff experience totals over 60 years in Clinical / Medical Research.
Our staff recruits for and manages studies in Utah, Nevada, and Colorado.